On October 15, 2022, the “World Journal of Diabetes” published the latest clinical research paper by Professor Zhang Fan and others from the Department of Endocrinology of Peking University Shenzhen Hospital,
This is one of the 111 stem cell dual-registration clinical research projects announced by the Chinese National Health Commission. The first study results on umbilical cord mesenchymal stem cells for the treatment of type 2 diabetes were released.
Type 2 diabetes: urgent solution
Diabetes has been one of the major public health problems worldwide for 100 years.
According to the latest data released by the International Diabetes Federation in 2022, the global prevalence of impaired glucose tolerance (IDF) in adults in 2021 is 9.1%, with a population of 464 million. It is expected that this proportion will increase to 10% by 2045, affecting 640 million adults. It is estimated that the prevalence rates of diabetes and prediabetes in Chinese adults are 10.9% and 35.7% respectively, of which type 2 diabetes accounts for more than 90%. In China, only 5.6% of patients with type 2 diabetes achieved blood sugar control in 2017.
Type 2 diabetes is considered a chronic progressive disease, which is caused by a relative decrease in insulin secretion due to functional defects of pancreatic β cells, or a decrease in insulin’s ability to metabolize glucose in the body due to insulin resistance (IR).
Current treatments for diabetes include diet control, physical exercise, oral antidiabetic drugs, and insulin therapy. Although new medications and dietary therapies are constantly being developed, none can completely prevent the degradation of beta cell function. Therefore, there remains an unmet need for an effective and safe strategy to restore β-cell function in patients with type 2 diabetes.
Many studies have shown that stem cells are a potentially feasible solution for the functional treatment of diabetes, but it still needs to be confirmed by a large number of multi-sample clinical trials.
Treatment and Cell Preparation
The clinical study isClinical research registration project of the National Health Commission jointly applied by Peking University Shenzhen Hospital and Beike Biotech, the project is: Umbilical cord mesenchymal stem cells were used to treat and follow up patients with type 2 diabetes and evaluate the safety and effectiveness of the treatment, where Umbilical cord mesenchymal stem cells were prepared and tested by Shenzhen Beike Biotechnology Co., Ltd.
This study was approved by the Institutional Review Board of Peking University Shenzhen Hospital (IRB Approval No. [2018] 29th)), and all patients signed informed consent.
16 selected patients (inclusion criteria were diagnosis of type 2 diabetes, age between 18 and 70 years old, HbA1c glycated hemoglobin level between 7% and 9.5%) received a total of three infusions of umbilical cord mesenchymal stem cells, one week apart between the three times, and each intravenous infusion dose was 1 × 10^6 cells per kilogram of body weight.
During the follow-up period, participants self-monitored fasting blood glucose and 2-hour postprandial blood glucose 7 times a week, while adjusting the dosage of oral hypoglycemic drugs and insulin according to the patient’s blood glucose to maintain stable levels.
All patients were medically evaluated again after 16 weeks.
Effectiveness evaluation
1. Significant improvement in blood sugar indicators
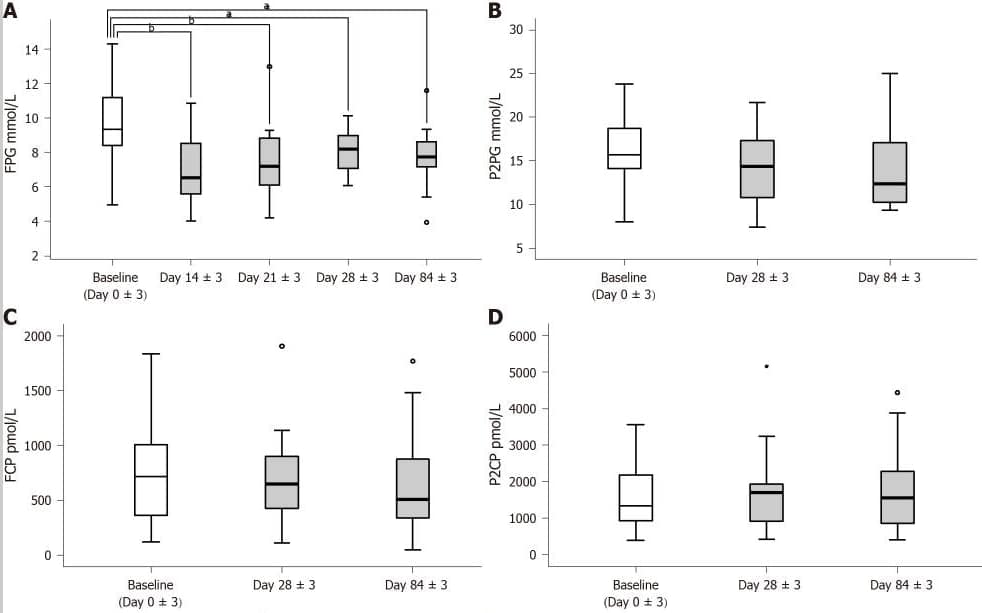
The patient’s fasting blood glucose (FPG) was significantly reduced on day 14±3, while the dose of antidiabetic drugs did not change and reached the lowest level during the entire intervention period.
During the follow-up period, with the reduction of antidiabetic drugs in all patients, fasting blood glucose FBG continued to decrease.
Glycated hemoglobin HbA1c levels decreased significantly on day 84±3.
A: Test fasting blood glucose on day 0±3, day 14±3, day 21±3, day 28±3 and day 84±3; B-D: Test 2-hour postprandial blood glucose, fasting C-peptide and 2-hour postprandial C-peptide on day 03, day 283 and day 843.
2. Improvement of pancreatic beta cell function
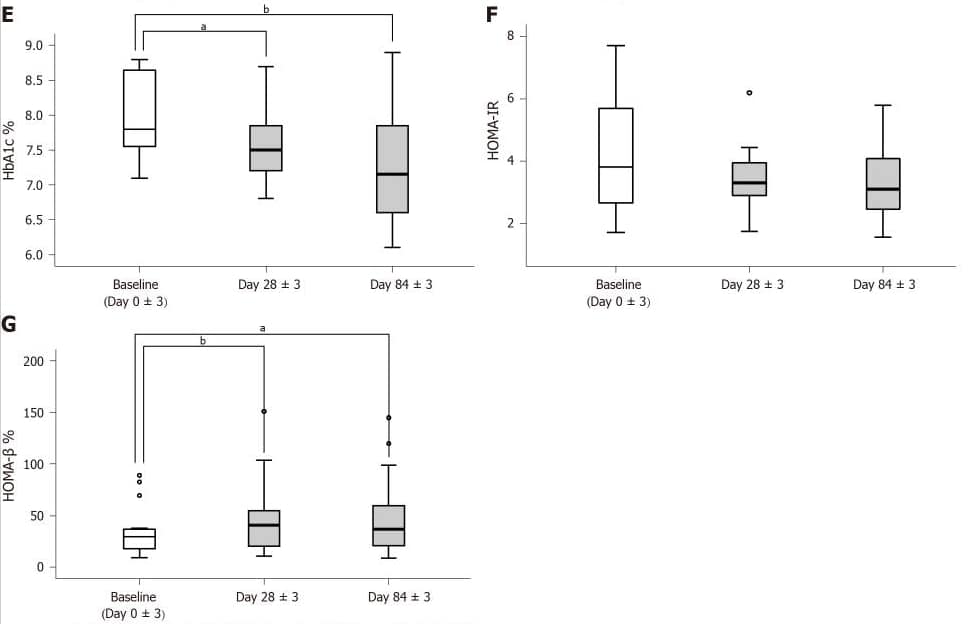
The patient’s islet β-cell function improved significantly on day 28±3.
Improvements in islet beta cell function (HOMA-β) stabilized and continued during the remainder of the intervention, and antidiabetic medications were reduced in all patients.
The insulin resistance index HOMA-IR decreased, but did not reach a statistically significant level.
E: Glycated hemoglobin A1c levels tested on days 0±3, 28±3 and 84±3; F and G: Homeostatic model assessment of insulin resistance and homeostatic model assessment of β-cell function calculated on days 03, 283 and 843.
3. Reduce the dosage of hypoglycemic drugs
After intravenous infusion of umbilical cord mesenchymal stem cells, the dosage of antidiabetic drugs was reduced in all patients on day 28±3, with 12 cases (75%) reducing it by 10%-50% and 4 cases (25%) reducing it by 50%.
On day 84±3, All patients had their dose reduced, with 6 (50%) patients achieving a reduction of more than 50% and 1 (6.25%) patient discontinuing antihyperglycemic agents.
Security assessment
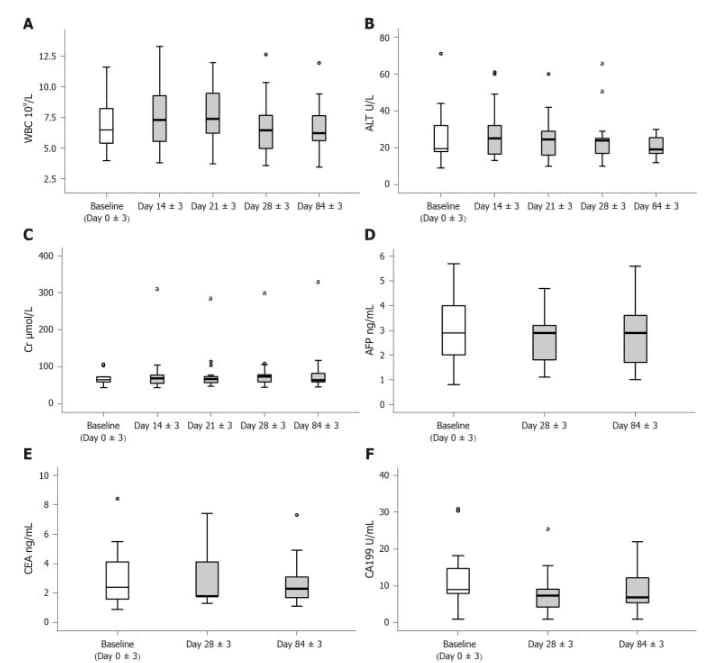
There was a transient significant increase in white blood cells on days 14 ± 3 after the first dose of umbilical cord mesenchymal stem cell treatment, but after the second dose, there was no significant difference in white blood cells compared with baseline. White blood cell levels remained stable over the following period. Serum levels of alanine aminotransferase and creatinine did not change significantly. Only 4 patients experienced transient fever (a total of 5 episodes), which occurred within 24 hours after the second or third infusion.
Safety evaluation of human umbilical cord mesenchymal stem cell therapy. A-C: White blood cells, liver and kidney function; D-F: Tumor-associated antigen
After the three doses of umbilical cord mesenchymal stem cells, CA199 and alpha-fetoprotein only decreased on day 28 ± 3, and carcinoembryonic antigens did not significantly change during the entire follow-up period.
Conclusion
The researchers said this is a preliminary exploratory study and will continue to follow up participants for up to 3 years in the future. At the same time, more participants will be recruited and healthy control groups will be set up to evaluate the clinical efficacy of this therapy for type 2 diabetes.
In short, the clinical findings show that Umbilical cord mesenchymal stem cell therapy can improve blood sugar, restore pancreatic islet beta cell function, and safely reduce the dose of hypoglycemic drugs required by patients.
Therefore, umbilical cord mesenchymal stem cell therapy may become a new treatment for type 2 diabetes.
References:
Lian XF, Lu DH, Liu HL, et al. Effectiveness and safety of human umbilical cord-mesenchymal stem cells for treating type 2 diabetes mellitus. World J Diabetes. 2022;13(10):877-887. doi:10.4239/wjd.v13.i10.877.