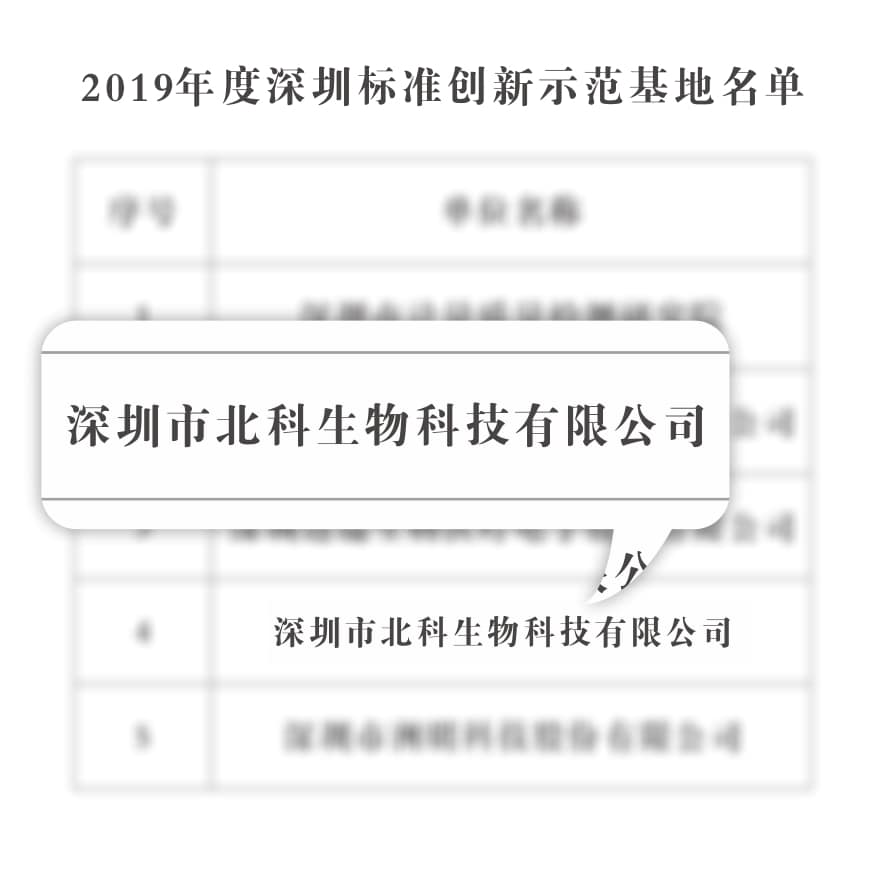
The Shenzhen Municipal Market Supervision and Administration Organization selected the Shenzhen Standard Innovation Demonstration Base in order to implement the innovation-driven development strategy, improve the full-chain standardized service model, and accelerate the construction of a technical standard innovation service system that meets the needs of technological innovation, industrial upgrading and economic and social development.To drive innovation and development of Shenzhen enterprises through the demonstration effect of leading enterprises.
The cell industry is related to life and health, and standardization work is particularly important. As a leader in this field, Beike Biotech has started the road to corporate standardization early.Referring to AABB’s management standards for cell therapy products, a complete internal enterprise standardized management has been formed. Through the combination of more than a dozen modules and more than 500 documents, coordinated operations have been completed to improve the closed loop of cell quality from donor to preparation to testing. inherit; font-size: inherit; line-height: inherit; font-family: inherit; vertical-align: baseline;”>Introducing standardized management from the R&D stage to ensure the authenticity and credibility of R&D results and the efficient and orderly R&D, transformation and production processes.
While building internal standardization work, Beike Biotechnology is also continuously promoting the standardization of the cell industry. It was jointly drafted by the Shenzhen Municipal Administration for Market Regulation, the Shenzhen Development and Reform Commission, Shenzhen Beike Biotechnology Co., Ltd. and the Shenzhen Institute of Standards and Technology“Cell Preparation Center Construction and Management Specifications” (No.: SZDB/Z 188-2016) has been officially released in June 2016; “Comprehensive Cell Bank Construction and Management Specifications” (No.: SZDB/Z 266-2017) was officially released in September 2017; it provides a useful reference for the formulation of industry standards for cell therapy technology in my country.
In the future, Beike Biotechnology, as the “Shenzhen Standardization Innovation Demonstration Base”, will continue to fulfill its responsibilities, continuously demonstrate the results of standardization innovation, promote more efficient development of enterprises, and exert its own strength in the process of group standards, industry, national and even international standards in the cell field, assisting the construction of “Shenzhen Standards” and promoting standardization work in the field of cell therapy technology throughout the Greater Bay Area.